Tag Archives: robotics
minimallyinvasive gastric surgery
Oggi 2 Febbraio 2015 ore 10.00 Aula robotica della nostra International School of Robotics in Grosseto ITA :il fervore delle grandi occasioni,chirurghi da tutta la Toscana,è attesa una lettura magistrale da parte di YW Kim massimo esperto di chirurgia gastrica mininvasiva ,giunto ieri da Seul.E’disinvolto,gentile ed affabile come lo sono molti asiatici,ma si percepisce un carattere determinato come attestato dalle 5000 procedure gastriche del suo curriculum.Una cifra da urlo.E l’incontro non tradisce le attese!
Emerging Role of Robot-assisted Gastrectomy: Analysis of Consecutive 200 Cases
Ji Yeon Park,1 Young-Woo Kim,corresponding author1 Keun Won Ryu,1 Bang Wool Eom,1 Hong Man Yoon,1 and Daniel Reim1,2
Author information ► Article notes ► Copyright and License information ►
This article has been cited by other articles in PMC.
Robotic surgery for gastric cancer is a promising alternative to laparoscopic surgery, but the data are limited. We aimed to evaluate whether gaining experience in robotic gastrectomy could improve surgical outcomes in the treatment of gastric cancer.
Materials and Methods
Two hundred and seven consecutive cases of patients with clinical stage I gastric cancer who underwent robotic surgery at the National Cancer Center of Korea between February 2009 and February 2012 were retrospectively reviewed. Surgical outcomes were analyzed and compared between the initial 100 and later 100 cases.
Results
Seven patients required conversion to open surgery and were excluded from further analysis. The mean operating time for all patients was 248.8 minutes, and mean length of hospitalization was 8.0 days. Twenty patients developed postoperative complications. Thirteen were managed conservatively, while 6 had major complications requiring invasive procedures. One mortality occurred owing to myocardial infarction. Operating time was significantly shorter in the latter 100 cases than in the initial 100 cases (269.9 versus 233.5 minutes, P<0.001). The number of retrieved lymph nodes was significantly greater in the latter cases (35.9 versus 39.9, P=0.032). The hospital stay of patients with complications was significantly longer in the initial cases than in the latter cases (16 versus 7 days, P=0.005).
Conclusions
Increased experience with the robotic procedure for gastric cancer was associated with improved outcomes, especially in operating time, lymph node retrieval, and shortened hospital stay of complicated patients. Further development of surgical techniques and technology might enhance the role of robotic surgery for gastric cancer.
Keywords: Stomach neoplasms, Minimally invasive surgical procedures, Robotics, Laparoscopy
Introduction
Robotic technology is one of the latest developments in minimally invasive surgery. Adoption of robotic surgery has come into the spotlight in various fields of surgery as a solution to the shortcomings of conventional laparoscopic surgery and is expected to play a role in advanced laparoscopic surgery. A robotic system was first applied to gastric cancer surgery in 1997, but reports on robotic gastrectomy are sparse owing to its slow adoption in many countries. After Song et al.1 reported on a large series of robotic surgeries for gastric cancer in 2005, the procedure gradually increased in popularity among several tertiary hospitals in Korea.
The most important clinical question regarding use of a robotic system vs. conventional open or laparoscopic surgery for gastric cancer is whether there is an objective benefit to compensate for its high expense. Laparoscopic gastrectomy has long been promoted for feasibility and efficacy for gastric cancer2-4 and ultimately has proven to be superior to open surgery in terms of postoperative quality of life, shortened hospital stay, and improved postoperative pain.5 Several studies have demonstrated that robotic gastrectomy is comparable to laparoscopic gastrectomy in terms of feasibility and safety, but few have shown a definite benefit of robotics over the laparoscopic approach, and most studies included their initial learning experience in the analysis.1,6-8 It would be easy to justify adopting robotic gastrectomy, in spite of its high cost, if there were any specific value. Considering the time it takes to master the standard operative technique with newly adopted instruments, an unbiased assessment of robotic gastrectomy after the initial learning experience is necessary.
This study investigated 200 consecutive cases of robotic gastrectomy in a single institution and compared the surgical outcomes between the initial 100 and later 100 cases. We aimed to evaluate the improvement in surgical outcomes associated with the learning experience of robot-assisted gastrectomy and to investigate the possible benefit of robotic assistance in gastric cancer surgery after sufficient experience.
Materials and Methods
1. Study design and inclusion criteria
A robotic system was first used for gastric cancer surgery at the National Cancer Center of Korea in February 2009. Between February 2009 and February 2012, 207 consecutive patients who were clinically diagnosed with stage I gastric cancer according to the American Joint Committee on Cancer (AJCC) 7th edition and underwent robot-assisted gastrectomy were enrolled in a retrospective analysis. Clinical diagnosis was obtained by esophagogastroduodenoscopy, endoscopic ultrasonography, and computed tomography. The prospectively established database and medical records of these patients were retrospectively reviewed.
2. Operative procedures
The da Vinci® Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA) was used for all robotic procedures. The procedures were performed by 3 surgeons who were all highly experienced in conventional laparoscopic gastrectomy. These surgeons all completed at least 200 laparoscopic gastrectomies prior to performing robotic surgery. The operation was performed according to the institutionally standardized operative rules: (1) partial omentectomy, (2) resection margin more than 2 cm from the primary tumor, and (3) D1+ or more lymphadenectomy based on Japanese Research Society for Gastric Cancer guidelines.
Patients were placed under general anesthesia and positioned in reverse Trendelenburg with slight leg elevation. The camera port was inserted through the umbilicus with a 12-mm trocar after establishing the pneumoperitoneum through a Veress needle. Three additional trocars (8 mm in diameter) for robotic arms were placed under camera visualization, and 1 assistant port was placed in the left umbilical level port using a 12-mm trocar. After docking the robotic arms with a surgical cart placed above the patient’s head, a modified liver lift was performed, enabling full exposure of the operative field by transfixing the left lateral segment of the liver to the abdominal wall with a straight needle. Four robotic arms were used during the operation: a central one for a dual-channel endoscope, and the others for Cadiere forceps, bipolar Maryland forceps, and unipolar electrocautery or ultrasonic shears, depending on the surgeon’s preference. The procedure itself was similar to conventional laparoscopic procedures described previously.8,9 After dissection and full mobilization of the stomach, the robotic arms were undocked from the patient. All anastomoses were performed extracorporeally via a 4 to 6 cm mini-laparotomy incision in the epigastrium.
3. Surgical and oncologic outcomes
Electronic medical records and prospectively collected data from the Gastric Cancer Center database were reviewed for surgical outcomes. Intraoperative parameters such as operating time and blood loss were assessed. Operating time was defined as time from skin incision to wound closure. Postoperative complications were graded according to the Accordion Severity Grading System.10 Pathologic results were also reviewed in terms of Lauren’s classification, histology, tumor location, size, and resection margin. Final pathologic stage was assessed according to the AJCC 7th edition.11
4. Statistics
Statistical analysis was performed using the SAS program (SAS Institute Inc., Cary, NC, USA). Means and standard deviations were calculated. The chi-square test or Fisher’s exact test was applied to analyze categorical variables, and the Student’s t-test was used for continuous variables. Linear regression was applied to evaluate change in operating time according to the accumulated cases. A P-value less than 0.05 was considered statistically significant.
Results
1. Patient characteristics and their surgical outcomes
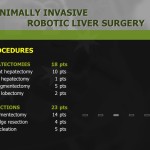
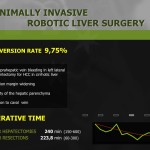
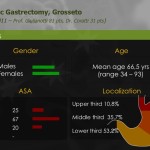
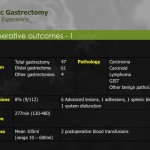
Seven patients required conversion to open surgery due to severe adhesion, bleeding during operation, or inadequate resection margin, and these patients were excluded from further analysis. The demographics of the 200 enrolled patients are shown in Table 1. The study patients consisted of 110 men and 90 women, and mean age of the patients was 53.4 years. Of the 200 total patients, 154 underwent subtotal gastrectomy, and 46 underwent total gastrectomy with more than D1+ lymph node dissection. The mean operating time for all patients was 248.8 minutes, and mean length of hospitalization was 8.0 days. Mean number of retrieved lymph nodes was 37.9. The final pathologic report revealed that 22 patients had cancers beyond stage II, and 31 patients showed lymph node metastasis. Resection margins were tumor-free in all patients (Table 2).
Table 1
Table 1
Demographics of the patients (n=200)
Table 2
Table 2
Surgical and pathologic outcomes
2. Postoperative complications
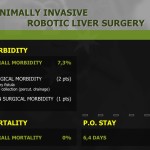
Twenty patients (10%) developed postoperative complications (Table 3). Half of them developed mild complications, such as wound problems, adhesive ileus, or delayed emptying, and 3 developed complications of moderate severity. All were successfully managed conservatively. Six patients had severe complications requiring invasive procedures, including 3 cases of reoperation: one duodenal stump repair, one hernia repair, and one open drainage for pancreatitis that did not respond to medical treatment. One mortality due to myocardial infarction occurred on postoperative day 2. No specific intraoperative events, such as bleeding or traumatic injury to normal organs, occurred.
Table 3
Table 3
Postoperative complications graded according to the Accordion severity classification (n=20)
3. Comparison of surgical outcomes between initial 100 and later 100 cases
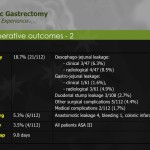
Surgical outcomes of the initial 100 and later 100 cases were reviewed and compared (Table 4). Operating time was significantly shorter in the late surgery group as compared to that in the early cases (269.9±54.8 vs. 233.5±51.3 minutes, P<0.001). The number of retrieved lymph nodes was greater in the latter 100 cases, and this difference was statistically significant (35.9±13.0 vs. 39.9±13.4, P=0.032). Blood loss during surgery and the incidence of postoperative complications did not differ between the 2 groups. Duration of hospital stay was shorter in the latter than in the earlier 100 cases, but the difference was not statistically significant (P=0.092). Mean length of hospital stay after surgery in complicated cases was compared between the 2 groups (Fig. 1). Although the incidence and severity distribution were not different between the 2 groups, the median length of stay of 8 patients with complications in the initial 100 cases was significantly longer than that of 12 patients in the latter 100 cases (16 vs. 7 days, P=0.005, Mann-Whitney U test). Subgroup analysis according to surgical extent showed similar results (Table 5). Operating time was significantly decreased in the latter 100 cases, both in subtotal and total gastrectomy (P<0.001 and =0.003, respectively). The number of retrieved lymph nodes was greater in the latter 100 cases, both in subtotal and total gastrectomy, but the difference was not statistically significant (P=0.243 and 0.091, respectively).
Fig. 1
Fig. 1
Comparison of length of hospital stay between the initial and latter experience in complicated cases. Error bar depicts interquartile ranges (Mann-Whitney U test applied).
Table 4
Table 4
Comparison between early and late surgical experiences
Table 5
Table 5
Comparison of surgical outcomes according to the surgical extent
4. Relationship between operating time and accumulation of experience
Operating time gradually decreased along with the accumulation of surgical experience in 2 participating surgeons (Fig. 2). For one surgeon who only performed robotic surgery in 11 cases, no significant correlation to improved operating time could be demonstrated. Operating time during subtotal gastrectomy was inversely related to the number of robotic surgeries performed and revealed statistical significance in both surgeon I and II (P<0.001 and P=0.037, respectively). Likewise, a decreasing trend in operating time was seen during total gastrectomy, but the relationship was statistically significant only in surgeon I (P=0.039).
Fig. 2
Fig. 2
Change in operating time. Operating time gradually decreased along with the accumulation of surgical experience. (A) Robot-assisted distal gastrectomy, (B) Robot-assisted total gastrectomy.
Discussion
Robotic gastrectomy is reported to be increasingly performed in Korea over the last 7 years.12 Nonetheless, evidence for the effectiveness of robotic surgery for gastric cancer is limited. Only several case series have been published so far, mostly by Eastern Asian centers,1,13-16 and prospectively randomized controlled trials (RCTs) reporting on safety and feasibility have not yet been published.12 By contrast, the value, safety, and feasibility of laparoscopic surgery for early gastric cancer has been proven in several RCTs.2,3,5,17 Robotic surgery is assumed to improve short-term patient outcomes such as length of postoperative hospital stay, frequency of postoperative complications, and quality of life.12 However, all previous studies have failed to demonstrate the superiority of robotic surgery as compared to the laparoscopic approach. Our previous study revealed that surgical trauma in terms of cytokine response was not reduced in robotic surgery patients as compared with laparoscopically resected patients.18
The main focus of our analysis was to see whether increasing experience with robotic gastrectomy could improve the quality of surgery after the known learning curve of 20 initial cases.19 We found that gaining experience with the robotic procedure translated into improved outcomes, especially in terms of operating time and number of dissected lymph nodes.
Mean operating time in our cohort was approximately 250 minutes, with a statistically significant decrease of operating time within the analyzed period. The first 100 cases had a mean operating time of around 270 minutes compared to approximately 230 minutes for the second 100 cases. Operating times in previously published series range from 150 to 700 minutes, depending on the type of resection.1,6-8,13-17,20-24 Interestingly, there appears to be a relationship between caseload and operating time. Institutions with low caseloads seem to take longer for the robotic procedure compared to the time taken by those with high caseloads where surgeons presumably have more experience with minimally invasive laparoscopic surgery. Compared to various published studies, our study cohort was among those with the shortest operative time, especially after the first 100 cases. It remains elusive if shorter operative time results in a lower postoperative complication rate. The prevailing mode of reconstruction in our analysis was Billroth I, followed by Billroth II and Roux-en-Y anastomosis, according to the surgeon’s preference. We did not notice any difference in operating time related to reconstruction methods as all anastomoses were performed extracorporeally. Nonetheless, intra-abdominal robot-sewn anastomosis has been demonstrated to be safe and feasible14 and might contribute to reduction of postoperative trauma in the future.
Blood loss within our cohort lies within the range of other published series.1,6-8,13-17,20-24 We also found a marked reduction in blood loss within our cohort after the first 100 cases of surgical experience, but it was not statistically significant. Reviewing published data, blood loss was almost always reduced for robotic surgery patients as compared to laparoscopic or open surgery patients.1,6-8,20 It may be debatable if application of robotic surgery really influences operative trauma, and consequently blood loss, or if due to the retrospective aspect of the studies, a selection bias of patients prevailed. A selection bias may have been that only patients with clinical stage IA cancer were selected for robotic surgery and limited lymphadenectomy was performed. Nonetheless, blood loss improved as patient numbers increased, so a learning curve is likely.25
We report a postoperative complication rate of 10%, which compared to previously published studies, is acceptable. European studies reported postoperative complication rates of up to 46%20,21,23 whereas Asian studies demonstrate postoperative complication rates of 10% to 15%.1,6-8,13-15,22,24 The high complication rates from European publications may be explained by the relatively lower caseload due to reduced gastric cancer incidence. It is also conceivable that less experience in minimally invasive (laparoscopic) surgery for gastric cancer may explain the higher European complication rates. The classification of complication might have been different as well, especially when the Accordion Severity Grading System was applied.10 Nonetheless, postoperative stay in the present study is among the shortest compared to other published studies. This might be, in part, due to the low postoperative morbidity rate of 10%. Furthermore, the median length of hospital stay for complicated patients was markedly decreased in the later 100 cases. This suggests that surgical outcomes regarding the severity of postoperative complications improve with the accumulation of experience in robotic gastrectomy.
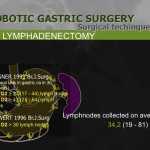
A crucial aspect of curative resection for gastric cancer patients is adequate lymphadenectomy. We found an increased number of dissected lymph nodes in the second 100 patients. Therefore, we believe that lymph node retrieval improves with increasing experience with the robotic procedure. Although the benefit of lymph node dissection in gastric cancer treatment is still controversial in Europe,26,27 improved lymph node retrieval could be an advantage of robot-assisted surgery. The 3-dimensional and other enhanced imaging techniques used in robotic surgery to identify lymph nodes may contribute to an improved oncologic outcome for patients.15 Within our patient cohort, a mean number of 38 lymph nodes were dissected, indicating appropriate lymphadenectomy as compared to previously published papers.1,6-8,13-17,20-24 Interestingly, European case series reveal lower numbers of resected nodes compared to Asian reports, suggesting that traditional experience with D2 dissection translates into successful application of the robotic procedure. When robot-assisted resection was compared to laparoscopic resection in previous reports, there was no inferiority of lymph node retrieval for the robotic procedure, indicating that adequate lymphadenectomy is safe and feasible in robotic gastrectomy.6-8,24
The present study has several limitations. It was based on a retrospective analysis, which could have led to a selection bias. One of the participating surgeons contributed only 11 cases out of 200, and his experience gained might be questionable. The surgical outcomes were not properly stratified according to the additional combined surgical procedures, such as cholecystectomy or splenectomy.
In conclusion, we found that increased experience with the robotic procedure for gastric cancer surgery translated into improved outcomes, especially in operating time and number of dissected lymph nodes. Prospective trials will have to focus on patient benefit and outcome. Improvements in lymphadenectomy may be conceivable due to the technical advantages of the robotic device compared to laparoscopic procedures
References
1. Song J, Kang WH, Oh SJ, Hyung WJ, Choi SH, Noh SH. Role of robotic gastrectomy using da Vinci system compared with laparoscopic gastrectomy: initial experience of 20 consecutive cases. Surg Endosc. 2009;23:1204–1211. [PubMed]
2. Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N. Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68–72. [PMC free article] [PubMed]
3. Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–237. [PMC free article] [PubMed]
4. Noshiro H, Shimizu S, Nagai E, Ohuchida K, Tanaka M. Laparoscopy-assisted distal gastrectomy for early gastric cancer: is it beneficial for patients of heavier weight? Ann Surg. 2003;238:680–685. [PMC free article] [PubMed]
5. Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. [PubMed]
6. Eom BW, Yoon HM, Ryu KW, Lee JH, Cho SJ, Lee JY, et al. Comparison of surgical performance and short-term clinical outcomes between laparoscopic and robotic surgery in distal gastric cancer. Eur J Surg Oncol. 2012;38:57–63. [PubMed]
7. Woo Y, Hyung WJ, Pak KH, Inaba K, Obama K, Choi SH, et al. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg. 2011;146:1086–1092. [PubMed]
8. Yoon HM, Kim YW, Lee JH, Ryu KW, Eom BW, Park JY, et al. Robot-assisted total gastrectomy is comparable with laparoscopically assisted total gastrectomy for early gastric cancer. Surg Endosc. 2012;26:1377–1381. [PubMed]
9. Lee SE, Kim YW, Lee JH, Ryu KW, Cho SJ, Lee JY, et al. Developing an institutional protocol guideline for laparoscopy-assisted distal gastrectomy. Ann Surg Oncol. 2009;16:2231–2236. [PubMed]
10. Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of surgical complications. Ann Surg. 2009;250:177–186. [PubMed]
11. Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. [PubMed]
12. Marano A, Hyung WJ. Robotic gastrectomy: the current state of the art. J Gastric Cancer. 2012;12:63–72. [PMC free article] [PubMed]
13. Isogaki J, Haruta S, Man-I M, Suda K, Kawamura Y, Yoshimura F, et al. Robot-assisted surgery for gastric cancer: experience at our institute. Pathobiology. 2011;78:328–333. [PubMed]
14. Hur H, Kim JY, Cho YK, Han SU. Technical feasibility of robot-sewn anastomosis in robotic surgery for gastric cancer. J Laparoendosc Adv Surg Tech A. 2010;20:693–697. [PubMed]
15. Uyama I, Kanaya S, Ishida Y, Inaba K, Suda K, Satoh S. Novel integrated robotic approach for suprapancreatic D2 nodal dissection for treating gastric cancer: technique and initial experience. World J Surg. 2012;36:331–337. [PubMed]
16. Anderson C, Ellenhorn J, Hellan M, Pigazzi A. Pilot series of robot-assisted laparoscopic subtotal gastrectomy with extended lymphadenectomy for gastric cancer. Surg Endosc. 2007;21:1662–1666. [PubMed]
17. Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report–a phase III multicenter, prospective, randomized Trial (KLASS Trial) Ann Surg. 2010;251:417–420. [PubMed]
18. Park JY, Jo MJ, Nam BH, Kim Y, Eom BW, Yoon HM, et al. Surgical stress after robot-assisted distal gastrectomy and its economic implications. Br J Surg. 2012;99:1554–1561. [PubMed]
19. Kang BH, Xuan Y, Hur H, Ahn CW, Cho YK, Han SU. Comparison of surgical outcomes between robotic and laparoscopic gastrectomy for gastric cancer: the learning curve of robotic surgery. J Gastric Cancer. 2012;12:156–163. [PMC free article] [PubMed]
20. Caruso S, Patriti A, Marrelli D, Ceccarelli G, Ceribelli C, Roviello F, et al. Open vs robot-assisted laparoscopic gastric resection with D2 lymph node dissection for adenocarcinoma: a case-control study. Int J Med Robot. 2011;7:452–458. [PubMed]
21. D’Annibale A, Pende V, Pernazza G, Monsellato I, Mazzocchi P, Lucandri G, et al. Full robotic gastrectomy with extended (D2) lymphadenectomy for gastric cancer: surgical technique and preliminary results. J Surg Res. 2011;166:e113–e120. [PubMed]
22. Lee HH, Hur H, Jung H, Jeon HM, Park CH, Song KY. Robot-assisted distal gastrectomy for gastric cancer: initial experience. Am J Surg. 2011;201:841–845. [PubMed]
23. Patriti A, Ceccarelli G, Bellochi R, Bartoli A, Spaziani A, Di Zitti L, et al. Robot-assisted laparoscopic total and partial gastric resection with D2 lymph node dissection for adenocarcinoma. Surg Endosc. 2008;22:2753–2760. [PubMed]
24. Huang KH, Lan YT, Fang WL, Chen JH, Lo SS, Hsieh MC, et al. Initial experience of robotic gastrectomy and comparison with open and laparoscopic gastrectomy for gastric cancer. J Gastrointest Surg. 2012;16:1303–1310. [PubMed]
25. Heemskerk J, van Gemert WG, de Vries J, Greve J, Bouvy ND. Learning curves of robot-assisted laparoscopic surgery compared with conventional laparoscopic surgery: an experimental study evaluating skill acquisition of robot-assisted laparoscopic tasks compared with conventional laparoscopic tasks in inexperienced users. Surg Laparosc Endosc Percutan Tech. 2007;17:171–174. [PubMed]
26. Jansen EP, Boot H, van de Velde CJ, van San dick J, Cats A, Verheij M. Can adjuvant chemoradiotherapy replace extended lymph node dissection in gastric cancer? Recent Results Cancer Res. 2012;196:229–240. [PubMed]
27. Brisinda G, Crocco A, Tomaiuolo P, Santullo F, Mazzari A, Vanella S. Extended or limited lymph node dissection? A gastric cancer surgical dilemma. Ann Surg. 2012;256:e30–e31. [PubMed]
le tavole e le figure sono consultabili liberamente nell’articolo originale recensito su pubmed

Quest’opera è distribuita con Licenza Creative Commons Attribuzione – Non commerciale 4.0 Internazionale.
update in chirurgia robotica

Quest’opera è distribuita con Licenza Creative Commons Attribuzione – Non commerciale – Non opere derivate 3.0 Italia.